
Breadcrumb
- Home
- Labs
- Foundational Labs
- Spectroscopy
- Part 3: Analyzing Spectra
Part 3: Analyzing Spectra
The spectra you are observing is due to the gas being heated and are examples of emission line spectra. It would be most ideal to perform this lab in complete darkness, as light from outside, overhead, and the computer screen will inevitably be collected by the fiber optics cable and contaminate your spectra. This isn’t too much of a concern for this lab, but it’s important to realize.
The spectral lines, like the emission lines produced by the gas being heated in the tubes, are caused by transitions of electrons within the atom. When an electron decays from an excited state, or higher energy level, it will produce an photon. Transitions of electrons between energy levels produce photons with discrete energies. For example, in Hydrogen, an electron that transitions between the third and second level will always produce a photon with a wavelength of 656 nm, where as a transition between the fourth and second level produces a photon with a wavelength of 486 nm.
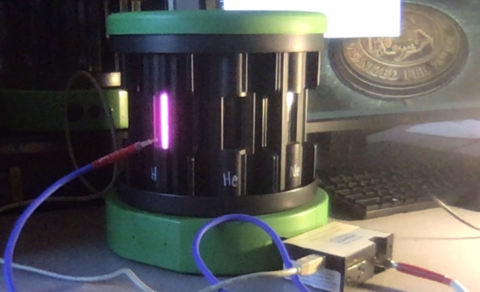
In this part of the lab, you will look at the emission spectra produced by samples of hot gas. The samples are installed in the Vernier spectrum tube carousel power supply, which is shown in the picture to the right. Spectra can be measured using the SpectraSuite software as in the previous section of the lab. The fiber optic cable of the spectrometer can be mounted in the plastic holder attached to the carousel.
There should be seven gas samples in the carousel: hydrogen, helium, nitrogen, neon, argon, carbon dioxide, and air. To move from one sample to the next, carefully rotate the middle part of the carousel until the next sample clicks into position. Look at the spectrum produced by each gas sample and discuss its features with your team, then use the information to answer the questions on your worksheet.
NOTE: The gas tubes can burn out if left on for extended periods. Remember to turn the unit off if you are not actively using it.