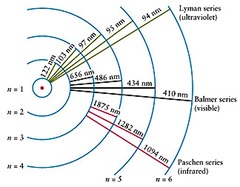
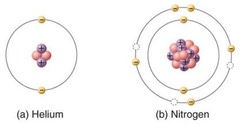
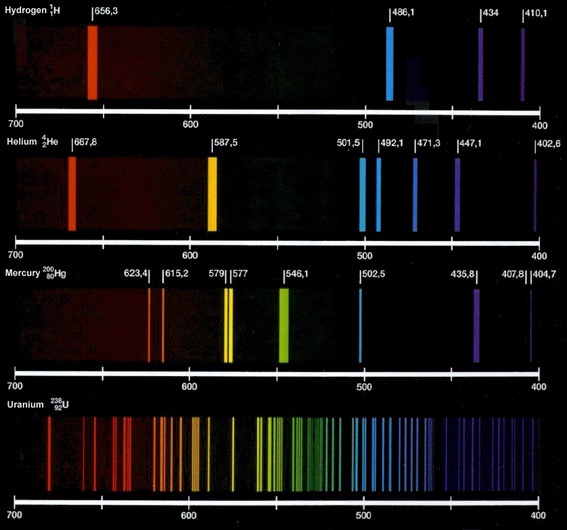
Learning Goals: The goal of this lab is to learn how a spectrum reveals the different frequencies present in a source of light, and how measuring the intensity of those frequencies can reveal things about the nature of the source. Students will learn the relationship between color and temperature and will see how the chemical composition of a source can be determined from its emission or absorption line spectrum.
Challenge: Understand the relationship between color and temperature for a light source. Identify a sample of hot gas by its pattern of emission lines.